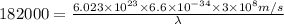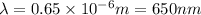The value of delta for the [c_rf_6]^3- complex is 182 kj/mol. calculate the expected wavelength of the absorption corresponding to promotion of an electron from the lower-energy to the higher-energy of orbital set in this complex. (remember to divide by avogadro's number.) should the complex absorb in the visible range?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
The value of delta for the [c_rf_6]^3- complex is 182 kj/mol. calculate the expected wavelength of t...
Questions




Mathematics, 04.03.2020 05:25

Mathematics, 04.03.2020 05:25





Mathematics, 04.03.2020 05:25


History, 04.03.2020 05:25



Mathematics, 04.03.2020 05:25





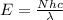
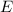 = energy of the wave = 182 kJ/mol = 182000 J/mol
= energy of the wave = 182 kJ/mol = 182000 J/mol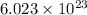
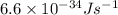
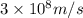
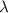 = wavelength of the wave = ?
= wavelength of the wave = ?