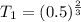Physics, 04.03.2020 05:25 daniellaZemira
Two thermally insulated cylinders, A and B, of equal volume, both equipped with pistons, are connected by a valve. Initially A has its piston fully withdrawn and contains a perfect monatomic gas at temperature T, while B has its piston fully inserted, and the valve is closed. Calculate the final temperature of the gas after the following operations, which each start with the same initial arrangement. The thermal capacity of the cylinders is to be ignored.
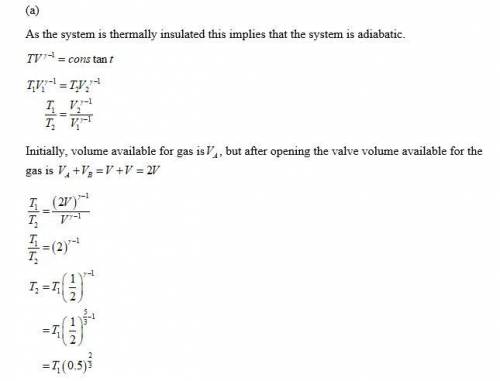
(a) The valve is fully opened and the gas slowly drawn into B by pulling out the piston B; piston A remains stationary.
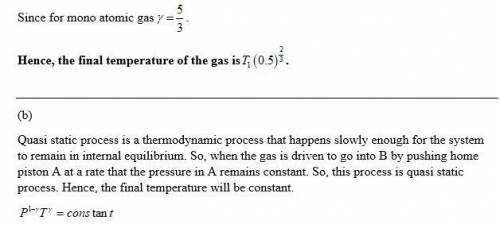
(b) Piston B is fully withdrawn and the valve is opened slightly; the gas is then driven as far as it will go into B by pushing home piston A at such a rate that the pressure in A remains constant: the cylinders are in thermal contact

Answers: 1


Another question on Physics


Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1

Physics, 22.06.2019 14:30
Will mark as brainliest how does a catapult increase the trajectory of an object? ps. answer as if u were a 5th grader
Answers: 1

You know the right answer?
Two thermally insulated cylinders, A and B, of equal volume, both equipped with pistons, are connect...
Questions


English, 07.12.2021 04:40

Social Studies, 07.12.2021 04:40

History, 07.12.2021 04:40



Mathematics, 07.12.2021 04:40

Mathematics, 07.12.2021 04:40


Mathematics, 07.12.2021 04:40



Biology, 07.12.2021 04:40

Mathematics, 07.12.2021 04:40

Health, 07.12.2021 04:40

Mathematics, 07.12.2021 04:40



Health, 07.12.2021 04:40

Biology, 07.12.2021 04:40