Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
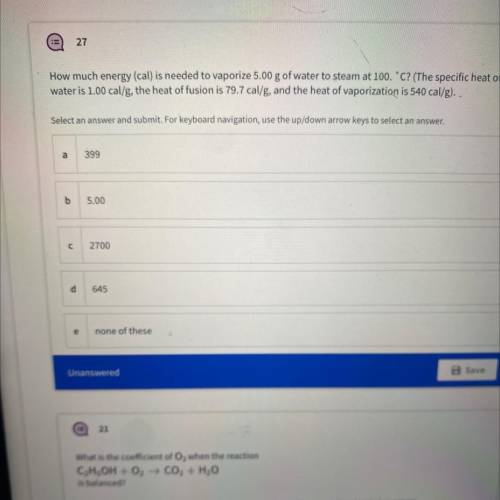
How much energy (cal) is needed to vaporize 5.00 g of water to steam at 100.C? (The specific heat of...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01