-
-
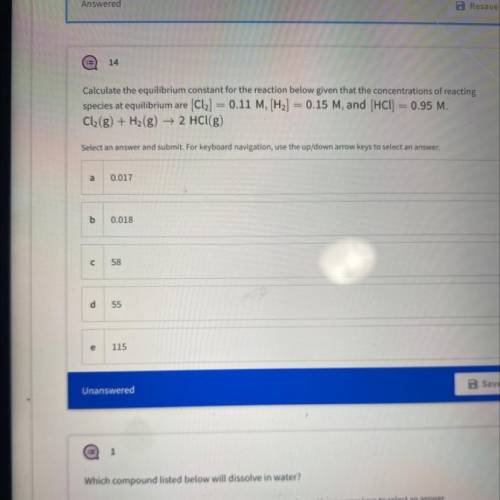
Calculate the equilibrium constant for the reaction below given that the concentrations...

Chemistry, 13.12.2021 06:40 ninigilford
-
-
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are [Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1


You know the right answer?
Questions


Biology, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14



Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14





Spanish, 10.04.2020 05:14




Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14



