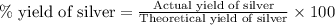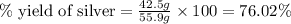Chemistry, 31.01.2020 07:04 rodriguezbrian050702
Astudent sets up a reaction and calculates a possible yield of 55.9 grams of silver. if the student performs the reaction and the mass of actual silver obtained is 42.5 grams, what is the percent yield?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Astudent sets up a reaction and calculates a possible yield of 55.9 grams of silver. if the student...
Questions




Social Studies, 31.12.2019 02:31