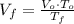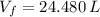Chemistry, 02.03.2021 14:00 kiingbr335yoqzaxs
A balloon filled with helium has a volume of 30.0 L at a pressure of 100 kPa and a temperature of 15.0 C. What will the volume of the balloon be if the temperature is increased to 80.0 C and the pressure remains constant? (Hint: Use the Charles law equation)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
A balloon filled with helium has a volume of 30.0 L at a pressure of 100 kPa and a temperature of 15...
Questions

History, 28.09.2019 06:50




Mathematics, 28.09.2019 06:50

Mathematics, 28.09.2019 06:50


Mathematics, 28.09.2019 06:50

Biology, 28.09.2019 06:50

History, 28.09.2019 06:50

Computers and Technology, 28.09.2019 06:50

Biology, 28.09.2019 06:50

English, 28.09.2019 06:50

Mathematics, 28.09.2019 06:50

Biology, 28.09.2019 06:50

Chemistry, 28.09.2019 06:50




Biology, 28.09.2019 06:50

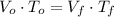 (1)
(1)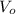 ,
, 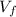 - Initial and final volume, measured in liters.
- Initial and final volume, measured in liters.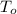 ,
, 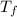 - Initial and final temperature, measured in Kelvin.
- Initial and final temperature, measured in Kelvin.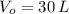 ,
, 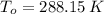 and
and 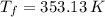 , the final volume of the balloon is:
, the final volume of the balloon is: