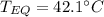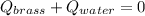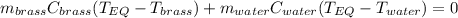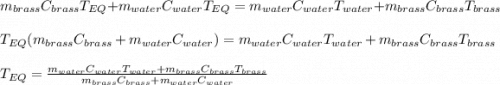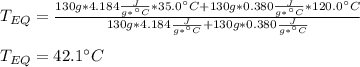Chemistry, 02.03.2021 14:00 LarryJoeseph
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g of water at 35.0 degrees Celsius. Disregard the absorption of heat by the cup and
calculate the final temperature of the brass and water. Specific heat of water = 4.18 J/gC,
specific heat of brass=0.380 J/gC. Attach your complete solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

You know the right answer?
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g...
Questions


English, 19.11.2020 07:00

History, 19.11.2020 07:00




English, 19.11.2020 07:00

Geography, 19.11.2020 07:00





Chemistry, 19.11.2020 07:00

History, 19.11.2020 07:00




Physics, 19.11.2020 07:00


Mathematics, 19.11.2020 07:00