
Chemistry, 19.06.2020 10:57 kcombest7219
Amylose is a "polysaccharide" that plants use to store energy. It is made of repeating subunits of C6H10O5. If a particular amylose molecule has 2537 of these subunits, what is its molecular formula? What is its molar mass? What is the empirical formula of amylose?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
Amylose is a "polysaccharide" that plants use to store energy. It is made of repeating subunits of C...
Questions











Computers and Technology, 04.09.2019 03:20








Computers and Technology, 04.09.2019 03:20

Mathematics, 04.09.2019 03:20

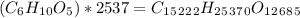
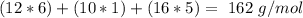
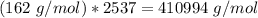
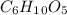 .
.

