A. +40 kJ

Chemistry, 05.05.2020 20:35 aarieejones
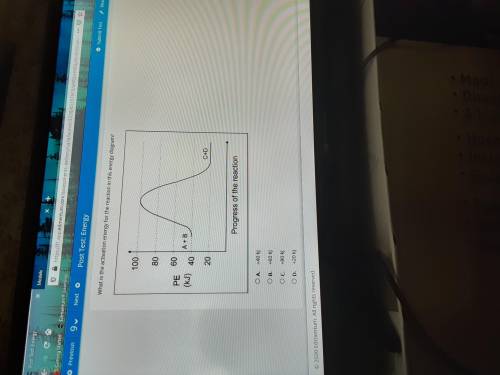
Plato: What is the activation energy for the reaction in this diagram?
A. +40 kJ
B. +60 kJ
C. +80 kJ
D. +20 kJ
E. +100 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1



Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Plato: What is the activation energy for the reaction in this diagram?
A. +40 kJ
A. +40 kJ
Questions

Geography, 20.09.2019 04:30

Mathematics, 20.09.2019 04:30


History, 20.09.2019 04:30





Mathematics, 20.09.2019 04:30

Mathematics, 20.09.2019 04:30


History, 20.09.2019 04:30



Physics, 20.09.2019 04:30


Physics, 20.09.2019 04:30

Chemistry, 20.09.2019 04:30

Computers and Technology, 20.09.2019 04:30



