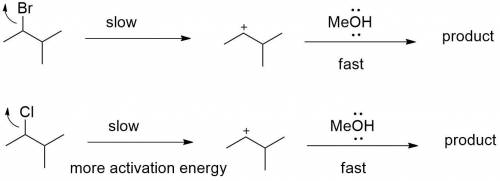
Chemistry, 05.05.2020 20:35 jacobp0712
Explain how the following changes would affect the rate of the reaction of 2-bromo-3-methylbutane with methanol: Part A The alkyl halide is changed to 2-chloro-3-methylbutane. The alkyl halide is changed to 2-chloro-3-methylbutane. The reaction will be slower because the leaving group will be poorer. The reaction will be faster because the leaving group will be better. The reaction will be slower because the leaving group will be better. The rate of the reaction does not change.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Explain how the following changes would affect the rate of the reaction of 2-bromo-3-methylbutane wi...
Questions


Mathematics, 07.05.2021 20:30

Chemistry, 07.05.2021 20:30






Social Studies, 07.05.2021 20:30


Physics, 07.05.2021 20:30



Social Studies, 07.05.2021 20:30




Mathematics, 07.05.2021 20:30

Biology, 07.05.2021 20:30

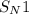 reaction.
reaction.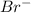 is a better leaving group than
is a better leaving group than 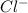 due to higher polarizability of
due to higher polarizability of