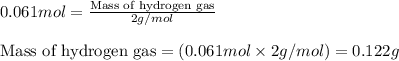Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2
You know the right answer?
How many grams of H2 can be produced by reaction of 1.80 g Al and 6.00 g
H2SO4?
2 Al + 3...
H2SO4?
2 Al + 3...
Questions








Mathematics, 30.04.2021 23:50


History, 30.04.2021 23:50

Mathematics, 30.04.2021 23:50

Physics, 30.04.2021 23:50



Health, 30.04.2021 23:50

Mathematics, 30.04.2021 23:50

Mathematics, 30.04.2021 23:50


English, 30.04.2021 23:50

Mathematics, 30.04.2021 23:50

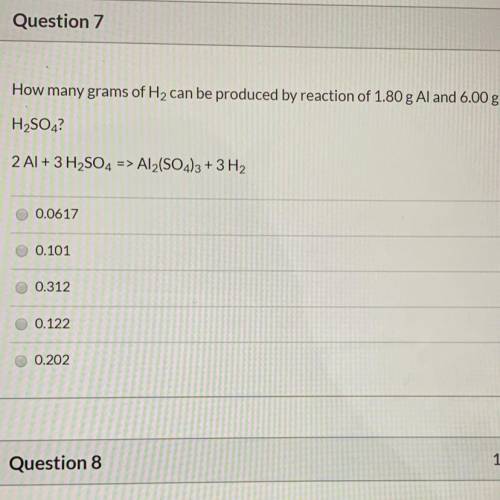
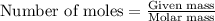 .....(1)
.....(1)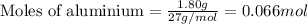
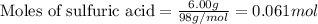
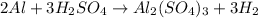
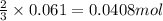 of aluminium
of aluminium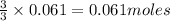 of hydrogen gas
of hydrogen gas