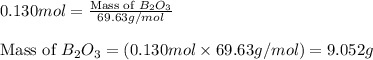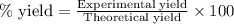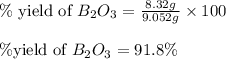Chemistry, 29.03.2020 21:45 demarpratt1270
Find percent yield:
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10.00 g of
O2 is 8.32 g. What is the percent yield?
2 B3H9 + 12 O2 => 5 B2O3 +9 H2O
87.2
92.8
91.8
75.5
74.5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
Find percent yield:
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10....
The mass of B2O3 produced by the reaction of 4.00 g of B5H9, and 10....
Questions

Social Studies, 05.05.2020 08:26

Mathematics, 05.05.2020 08:26



Arts, 05.05.2020 08:26





Mathematics, 05.05.2020 08:26


Mathematics, 05.05.2020 08:26

English, 05.05.2020 08:26






English, 05.05.2020 08:26

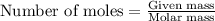 .....(1)
.....(1)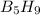 :
: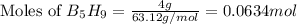
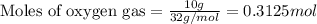
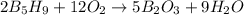
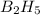
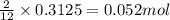 of
of 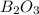
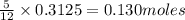 of water
of water