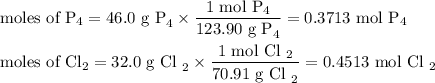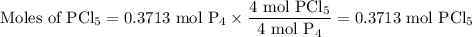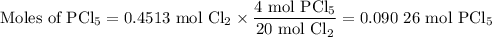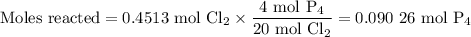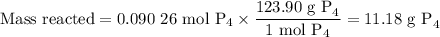Chemistry, 28.03.2020 04:02 shinyelish6
04.05 mol
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phosphorus pentachloride. When 32.0 g of chlorine reacts with 46.0 g of P4.
What is the mass in excess of the excess reactantt?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
04.05 mol
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phospho...
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phospho...
Questions

Business, 16.04.2021 17:30




English, 16.04.2021 17:30


Mathematics, 16.04.2021 17:30


Chemistry, 16.04.2021 17:30



Mathematics, 16.04.2021 17:30


English, 16.04.2021 17:30



English, 16.04.2021 17:30


Advanced Placement (AP), 16.04.2021 17:30

Mathematics, 16.04.2021 17:30