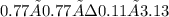A mixture containing initially 3.90 mole of NO(g) and 0.88 mole of CO2(g) was allowed to react in a flask of volume 1.00 L at a certain temperature according to the reaction CO2(g) + NO(g) ↔ CO(g) + NO2(g) At equilibrium 0.11 mole of CO2(g) was found present in the reaction mixture. Calculate the equilibrium constant Kc for the reaction at the temperature of the experiment.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
A mixture containing initially 3.90 mole of NO(g) and 0.88 mole of CO2(g) was allowed to react in a...
Questions





Physics, 18.02.2020 20:47

Mathematics, 18.02.2020 20:47


Social Studies, 18.02.2020 20:47


Health, 18.02.2020 20:48


Chemistry, 18.02.2020 20:48




English, 18.02.2020 20:48

Health, 18.02.2020 20:48

Mathematics, 18.02.2020 20:48


Mathematics, 18.02.2020 20:48

![\frac{[CO] *[NO2]} {[CO2]*[NO]}](/tpl/images/0562/7676/90ab2.png)