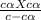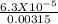Chemistry, 25.03.2020 06:39 aangellexith2885
Benzoic acid is a weak monoprotic acid. It has a pKa of 4.20. A student transferred 25.00mL of 0.126M benzoic acid into a beaker. The student then added 20.00mL of distilled water into the beaker. Calculate the equilibrium pH for this benzoic acid solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2


Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Benzoic acid is a weak monoprotic acid. It has a pKa of 4.20. A student transferred 25.00mL of 0.126...
Questions

Mathematics, 07.10.2019 10:50


Mathematics, 07.10.2019 10:50



Mathematics, 07.10.2019 10:50

Mathematics, 07.10.2019 10:50

English, 07.10.2019 10:50

History, 07.10.2019 10:50



Biology, 07.10.2019 10:50

Biology, 07.10.2019 10:50

Mathematics, 07.10.2019 10:50

History, 07.10.2019 10:50


Mathematics, 07.10.2019 10:50


History, 07.10.2019 10:50