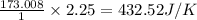Chemistry, 21.03.2020 04:26 zitterkoph
Consider the reaction 2CO(g) + O2(g)2CO2(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.25 moles of CO(g) react at standard conditions. S°surroundings = J/K

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
Consider the reaction 2CO(g) + O2(g)2CO2(g) Using standard thermodynamic data at 298K, calculate the...
Questions

Mathematics, 07.08.2019 04:30

Social Studies, 07.08.2019 04:30






Mathematics, 07.08.2019 04:30


Physics, 07.08.2019 04:30


Health, 07.08.2019 04:30




History, 07.08.2019 04:30




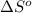 for the surrounding when given amount of CO is reacted is 432.52 J/K
for the surrounding when given amount of CO is reacted is 432.52 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0557/2961/52737.png)
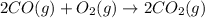
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(CO_2(g))})]-[(1\times \Delta S^o_{(O_2(g))})+(2\times \Delta S^o_{(CO(g))})]](/tpl/images/0557/2961/30479.png)
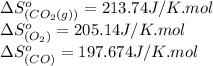
![\Delta S^o_{rxn}=[(2\times (213.74))]-[(1\times (205.14))+(2\times (197.674))]\\\\\Delta S^o_{rxn}=-173.008J/K](/tpl/images/0557/2961/a0064.png)