
Chemistry, 21.03.2020 04:33 samantha9014
The colorless gas phosphorus trifluoride reacts slowly with water to give a mixture of phosphorus acid and hydrofluoric acid . (a) Write a balanced chemical equation for this reaction. + + (b) Determine the concentration (in moles per liter) of each of the acids that result from the complete reaction of 5.71×10-2 moles of phosphorus trifluoride with enough water to give a solution volume of 711 mL. phosphorus acid : M hydrofluoric acid : M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
The colorless gas phosphorus trifluoride reacts slowly with water to give a mixture of phosphorus ac...
Questions


Mathematics, 26.08.2021 20:20





Mathematics, 26.08.2021 20:20


Mathematics, 26.08.2021 20:20

Business, 26.08.2021 20:20

Mathematics, 26.08.2021 20:20




Mathematics, 26.08.2021 20:20

History, 26.08.2021 20:20


History, 26.08.2021 20:20

Mathematics, 26.08.2021 20:20

Mathematics, 26.08.2021 20:20

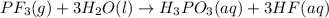
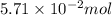
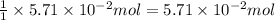 of phosphorus acid
of phosphorus acid![[concentration]=\frac{Moles}{Volume(L)}](/tpl/images/0557/3028/5aba9.png)
![[H_3PO_3]=\frac{5.71\times 10^{-2} mol}{0.711 L}=0.0803 M](/tpl/images/0557/3028/5fed3.png)
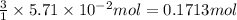 of hydrofluoric acid
of hydrofluoric acid![[HF]=\frac{0.1713 mol}{0.711 L}=0.241 M](/tpl/images/0557/3028/4f9ef.png)


