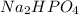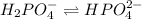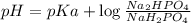Chemistry, 20.03.2020 11:19 MarishaTucker
Buffer consists of 0.50 M NaH2PO4 and 0.40 M Na2HPO4. Phosphoric acid is a triprotic acid (K a 1 = 7.2 × 10 − 3, K a 2 = 6.3 × 10 − 8, and K a 3 = 4.2 × 10 − 13). (a) Which Ka value is most important to this buffer? (b) What is the buffer pH?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2


Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Buffer consists of 0.50 M NaH2PO4 and 0.40 M Na2HPO4. Phosphoric acid is a triprotic acid (K a 1 = 7...
Questions


English, 30.01.2020 20:43







Physics, 30.01.2020 20:43




Biology, 30.01.2020 20:43


Biology, 30.01.2020 20:43

Mathematics, 30.01.2020 20:43

Mathematics, 30.01.2020 20:43


History, 30.01.2020 20:43

Mathematics, 30.01.2020 20:43

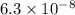 pKa2 = 7.2
pKa2 = 7.2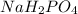 and
and