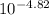Chemistry, 20.03.2020 11:22 GreenHerbz206
2. Acidity and basicity of solutions of common substances at 25 ºC: a. Calculate the pH of a sample of lemon juice with [H+ ] = 3.8 x 10–4 M. b. Calculate the pH of a commonly available window-cleaning solution with [OH– ] = 1.9 x 10–6 M. c. A sample of freshly pressed apple juice has a pH of 3.76. What is the [H+ ]? d. A solution formed by dissolving an antacid tablet has a pH of 9.18. What is the [OH– ]?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1


Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
2. Acidity and basicity of solutions of common substances at 25 ºC: a. Calculate the pH of a sample...
Questions



History, 12.12.2019 06:31

Mathematics, 12.12.2019 06:31




History, 12.12.2019 06:31




Mathematics, 12.12.2019 06:31

Mathematics, 12.12.2019 06:31



Mathematics, 12.12.2019 06:31

Chemistry, 12.12.2019 06:31


English, 12.12.2019 06:31