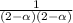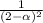Chemistry, 20.03.2020 11:19 mandilynn22
Kc = 9.7 at 900 K for the reaction NH3(g) + H2S(g) → NH4HS(s). If the initial concentrations of NH3(g) and H2S(g) are 2.0 M, what is the equilibrium concentration of NH3(g)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

You know the right answer?
Kc = 9.7 at 900 K for the reaction NH3(g) + H2S(g) → NH4HS(s). If the initial concentrations of NH3(...
Questions













History, 24.12.2019 23:31



Mathematics, 24.12.2019 23:31




![\frac{[NH4HS]/[NH3]}{[H2S]}](/tpl/images/0556/0364/b40c0.png) [Concentration of Solid NH₄HS = 1]
[Concentration of Solid NH₄HS = 1]