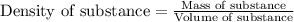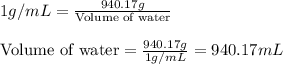Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2


Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
The solubility of in water at a certain temperature is 35.7 g /100. g . Suppose that you have 330.0...
Questions

Mathematics, 08.04.2021 01:50

Health, 08.04.2021 01:50

Mathematics, 08.04.2021 01:50

Biology, 08.04.2021 01:50





Mathematics, 08.04.2021 01:50


Mathematics, 08.04.2021 01:50

Mathematics, 08.04.2021 01:50


Advanced Placement (AP), 08.04.2021 01:50




English, 08.04.2021 01:50


Social Studies, 08.04.2021 01:50

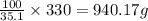 of water
of water