Chemistry, 11.03.2020 17:09 sylaspotter707
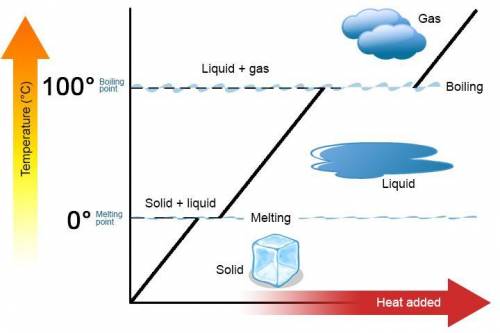
The specific heat of liquid ethanol, C2H5OH(l) is 2.46j/g degree celsius and the heat of vaporization is 39.3 kj/mol the boiling point of ethanol is 78.3 c what amount of etnthalpy is required to heat 50 g of liquid ethanol form 23 c to ethanol vapor a 78.3 c?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
The specific heat of liquid ethanol, C2H5OH(l) is 2.46j/g degree celsius and the heat of vaporizatio...
Questions

English, 14.12.2020 16:30








English, 14.12.2020 16:30




Mathematics, 14.12.2020 16:30

Mathematics, 14.12.2020 16:30




History, 14.12.2020 16:30


Mathematics, 14.12.2020 16:30

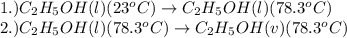
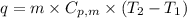
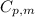 = specific heat capacity of liquid ethanol = 2.46 J/g°C
= specific heat capacity of liquid ethanol = 2.46 J/g°C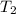 = final temperature = 78.3°C
= final temperature = 78.3°C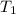 = initial temperature = 23°C
= initial temperature = 23°C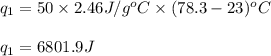
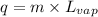
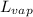 = latent heat of vaporization = 39.3 kJ/mol
= latent heat of vaporization = 39.3 kJ/mol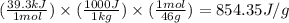
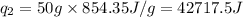
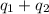
![[6801.9+42717.5]J=49519.4J=49.52kJ](/tpl/images/0542/9395/07659.png)