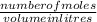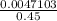A chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out ×4.7102μmol of silver(I) nitrate into a 450.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /mmolL of the chemist's silver(I) nitrate solution. Be sure your answer has the correc

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
A chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out ×4.7102μmol of silver(I) n...
Questions


Chemistry, 25.08.2020 01:01


Mathematics, 25.08.2020 01:01


Social Studies, 25.08.2020 01:01

Mathematics, 25.08.2020 01:01

Mathematics, 25.08.2020 01:01

Mathematics, 25.08.2020 01:01

Mathematics, 25.08.2020 01:01


Mathematics, 25.08.2020 01:01

Mathematics, 25.08.2020 01:01



Mathematics, 25.08.2020 01:01

Mathematics, 25.08.2020 01:01

Physics, 25.08.2020 01:01


Mathematics, 25.08.2020 01:01