Chemistry, 07.03.2020 04:28 pinapunapula
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The unbalanced equation for this reaction iCH4(g)+O2(g)→CO2(g)+H2O(g)This type of reaction is referred to as a complete combustion reaction. A)What mass of carbon dioxide is produced from the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units. B)What mass of water is produced from the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units. C)What mass of oxygen is needed for the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

You know the right answer?
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The unbalan...
Questions



Mathematics, 07.01.2020 23:31











Social Studies, 07.01.2020 23:31

Social Studies, 07.01.2020 23:31


Social Studies, 07.01.2020 23:31

Computers and Technology, 07.01.2020 23:31


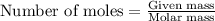 .....(1)
.....(1)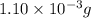
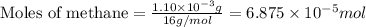
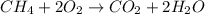
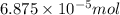 of methane will produce =
of methane will produce = 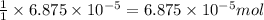 of carbon dioxide
of carbon dioxide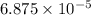 moles
moles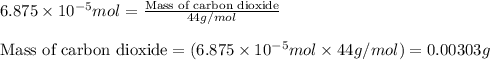
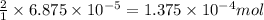 of water
of water moles
moles