Chemistry, 07.03.2020 04:54 cjstablet04
Complete and balance the molecular equation, including phases, for the reaction of aqueous copper (ll) chloride, CuCl2, and aqueous potassium phosphate, K3PO4.
Cu Cl,(aq) K, PO4(aq) -->
Enter the balanced net ionic equation, including phases, for this reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Complete and balance the molecular equation, including phases, for the reaction of aqueous copper (l...
Questions

Mathematics, 11.01.2021 19:30

SAT, 11.01.2021 19:30



Spanish, 11.01.2021 19:30




Mathematics, 11.01.2021 19:30







Advanced Placement (AP), 11.01.2021 19:30


Biology, 11.01.2021 19:30

History, 11.01.2021 19:30

Health, 11.01.2021 19:30

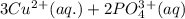 ⇒
⇒ 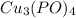
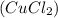 Tribasic Potassium Phosphate
Tribasic Potassium Phosphate 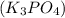
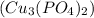 Potassium Chloride
Potassium Chloride 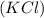
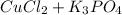 =
= 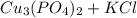
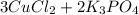 =
=