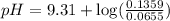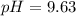Chemistry, 07.03.2020 04:54 andrew6494
A buffer solution contains 0.496 M hydrocyanic acid and 0.399 M sodium cyanide . If 0.0461 moles of sodium hydroxide are added to 225 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume does not change upon adding sodium hydroxide. )

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 14:00
Comparing john newland’s octaves with the modern periodic table, which 5 elements have been discovered between hydrogen and iron since newland’s time?
Answers: 3
You know the right answer?
A buffer solution contains 0.496 M hydrocyanic acid and 0.399 M sodium cyanide . If 0.0461 moles of...
Questions



Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00

Biology, 14.12.2020 21:00


Chemistry, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00

Mathematics, 14.12.2020 21:00


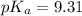
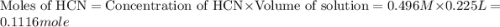
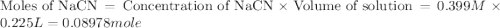
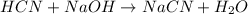
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0537/7961/e961a.png)