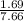Chemistry, 07.03.2020 02:47 garretthyatt123
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 200. mL flask with 2.6 atm of sulfur dioxide gas and 0.90 atm of oxygen gas at 35.°C. She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 1.3 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions


Mathematics, 24.06.2021 17:00

Mathematics, 24.06.2021 17:00

English, 24.06.2021 17:00


History, 24.06.2021 17:00

Mathematics, 24.06.2021 17:00






Computers and Technology, 24.06.2021 17:00

History, 24.06.2021 17:00

Mathematics, 24.06.2021 17:00



Mathematics, 24.06.2021 17:00


Mathematics, 24.06.2021 17:00

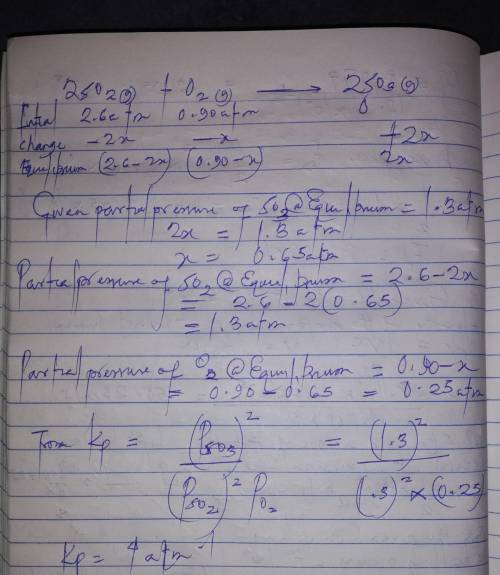
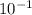 = 0.22 to 2 sig. fig.
= 0.22 to 2 sig. fig.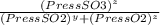
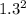 ÷ (
÷ (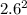 + 0.9)
+ 0.9)