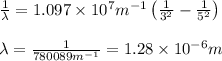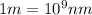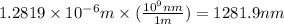Chemistry, 07.03.2020 02:47 mailani12503
Recall Planck's constant equals 6.63 × 10−34 J·s and the speed of light is 3.00 × 108 m/s. Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

You know the right answer?
Recall Planck's constant equals 6.63 × 10−34 J·s and the speed of light is 3.00 × 108 m/s. Calculate...
Questions

Mathematics, 30.06.2019 22:30


Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30

History, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30


Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30


History, 30.06.2019 22:30


Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30



Mathematics, 30.06.2019 22:40

History, 30.06.2019 22:40

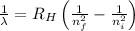
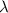 = Wavelength of radiation
= Wavelength of radiation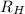 = Rydberg's Constant =
= Rydberg's Constant = 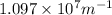
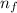 = Upper energy level = 3
= Upper energy level = 3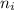 = Lower energy level = 5
= Lower energy level = 5