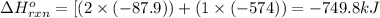Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Consider the following thermochemical equations.
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9k...
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9k...
Questions

Mathematics, 13.05.2021 22:10


Mathematics, 13.05.2021 22:10

Mathematics, 13.05.2021 22:10

Mathematics, 13.05.2021 22:10



Biology, 13.05.2021 22:20




Geography, 13.05.2021 22:20





Mathematics, 13.05.2021 22:20

Mathematics, 13.05.2021 22:20


Mathematics, 13.05.2021 22:20

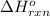 for the reaction is -749.8 kJ.
for the reaction is -749.8 kJ.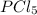 follows:
follows: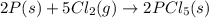
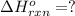
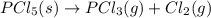
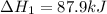 ( × 2)
( × 2)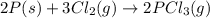
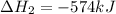
![\Delta H^o_{rxn}=[2\times (-\Delta H_1)]+[1\times \Delta H_2]](/tpl/images/0530/6491/d8429.png)