
Chemistry, 02.03.2020 21:03 liljobe8973
C6H5NH3Cl is a chloride salt with an acidic cation. If 46.3 g of C6H5NH3Cl is dissolved in water to make 150 mL of solution, what is the initial molarity of the cation

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
C6H5NH3Cl is a chloride salt with an acidic cation. If 46.3 g of C6H5NH3Cl is dissolved in water to...
Questions


Mathematics, 28.10.2020 19:20



Business, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Biology, 28.10.2020 19:20

Biology, 28.10.2020 19:20

English, 28.10.2020 19:20


Mathematics, 28.10.2020 19:20

Social Studies, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

History, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Biology, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20

English, 28.10.2020 19:20

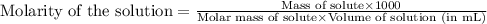
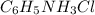 = 46.3 g
= 46.3 g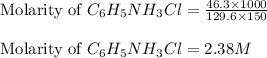
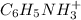 cation and 1 mole of
cation and 1 mole of 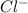 anion
anion

