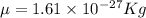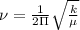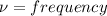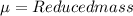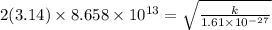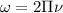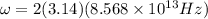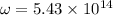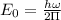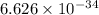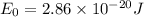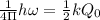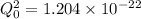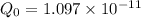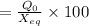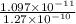Chemistry, 20.12.2019 21:31 tamyrareaves12
The hcl equilibrium bond length is 0.127 nm and the v = 0 to v = 1 transition is observed in the infrared at 2886 cm-1.compute the vibrational energy of hcl in its lowest state. compute the classical limit for the stretching of the hcl bond from its equilibrium length in this state. what percent of the equilibrium bond length is this extension

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
The hcl equilibrium bond length is 0.127 nm and the v = 0 to v = 1 transition is observed in the inf...
Questions


Mathematics, 14.12.2020 22:50



Mathematics, 14.12.2020 22:50

Mathematics, 14.12.2020 22:50


Mathematics, 14.12.2020 22:50



Mathematics, 14.12.2020 22:50

English, 14.12.2020 22:50

Mathematics, 14.12.2020 22:50


Mathematics, 14.12.2020 22:50

History, 14.12.2020 22:50


English, 14.12.2020 22:50


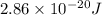
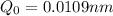
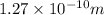
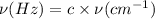

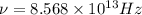
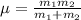
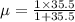
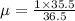
 and 1000(to convert gram itno Kg)
and 1000(to convert gram itno Kg)