Chemistry, 20.12.2019 21:31 petroale000
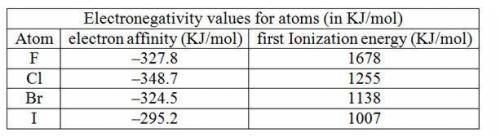
An alternative definition of electronegativity is electronegativity= constant (i. e — e. a.) where i. e. is the ionization energy and e. a. is the electron affinity using the sign conventions of this book. use data in chapter 12 to calculate the (i. e. - e. a.) term for f, cl, br, and i. do these values show the same trend as the electronegativity values given in this chapter? the first ionization energies of the halogens are 1678, 1255, 1138, and 1007 kj/mol, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
An alternative definition of electronegativity is electronegativity= constant (i. e — e. a.) where i...
Questions

Mathematics, 25.01.2020 05:31



Mathematics, 25.01.2020 05:31

English, 25.01.2020 05:31

Biology, 25.01.2020 05:31



Mathematics, 25.01.2020 05:31











![4=constant[1678-(-327.8)]](/tpl/images/0428/1473/7fe37.png)
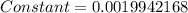
![Electronegativity=0.0019942168[1255+348.7]=3.1980\sim 3](/tpl/images/0428/1473/aa358.png)
![Electronegativity=0.0019942168[1138+324.5]=2.91\sim 2.9](/tpl/images/0428/1473/6b430.png)
![Electronegativity=0.0019942168[1007+295.7]=2.59\sim 2.5](/tpl/images/0428/1473/4bd84.png)