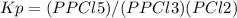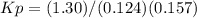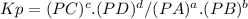Chemistry, 20.12.2019 21:31 BLASIANNkidd
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction:
pcl3 (g) + cl2 (g) → pcl5 (g)
an equilibrium mixture at 450 k contains ppcl3 = 0.124 atm, pcl2 = 0.157 atm, and ppcl5 = 1.30 atm.
what is the value of kp at this temperature?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1


Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlor...
Questions


Social Studies, 07.04.2021 23:30

English, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30



Biology, 07.04.2021 23:30

English, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30








Biology, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30


Mathematics, 07.04.2021 23:30