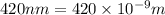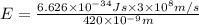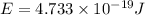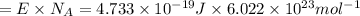Chemistry, 20.12.2019 18:31 pablogonzaleztellez
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 + hv > > > no(g) + o(g) the maximum wavelength of light that can cause this reaction is 420 nm. a) in what part of the electromagnetic spectrum is light with this wavelength found? b) what is the maximum strength of a bond, in kj/mol, that can be broken by absorption of a photon of 420-nm light? c) write out the photodissociation reaction showing lewis-dot structures.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

You know the right answer?
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 +...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








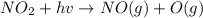
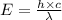
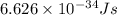
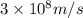
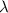 = wavelength =
= wavelength =