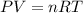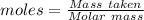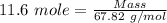Chemistry, 20.12.2019 18:31 hannabeth91
Boron trifluoride gas is collected at 2.0 degree c in an evacuated flask with a measured volume of 15.0 l. when all the gas has been collected, the pressure in the flask is measured to be 0.130 atm. calculate the mass and number of moles of boron trifluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
Boron trifluoride gas is collected at 2.0 degree c in an evacuated flask with a measured volume of 1...
Questions

Mathematics, 15.11.2020 20:10



Mathematics, 15.11.2020 20:10

Mathematics, 15.11.2020 20:10

Mathematics, 15.11.2020 20:10

Social Studies, 15.11.2020 20:10


Biology, 15.11.2020 20:10

World Languages, 15.11.2020 20:10









Spanish, 15.11.2020 20:20