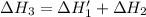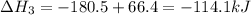Chemistry, 30.11.2019 01:31 tstaples02
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to create nitrogen dioxide. 2no (g) + o2 (g) → 2no2 (g)
calculate the standard reaction enthalpy for the above reaction using the following thermodynamic data.
n2 (g) + o2 (g) → 2no (g) ∆h˚1 = 180.5 kj
n2 (g) + 2o2 (g) → 2no2 (g) ∆h˚2 = 66.4 kj
-252.4 kj/mol rxn
-114.1 kj/mol rxn
-100.3 kj/mol rxn
-246.9 kj/mol rxn

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3


Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to cr...
Questions


Mathematics, 03.09.2019 04:10

Mathematics, 03.09.2019 04:10

Biology, 03.09.2019 04:10

Mathematics, 03.09.2019 04:10

Chemistry, 03.09.2019 04:10

Mathematics, 03.09.2019 04:10


Mathematics, 03.09.2019 04:10

Chemistry, 03.09.2019 04:10

English, 03.09.2019 04:10

Health, 03.09.2019 04:10

Chemistry, 03.09.2019 04:10







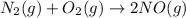 (1)
(1) 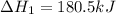
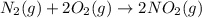 (2)
(2) 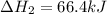
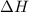 for the following reaction i.e,
for the following reaction i.e, (3)
(3) 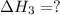
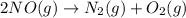 (1')
(1') 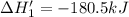
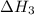 for the reaction will be:
for the reaction will be: