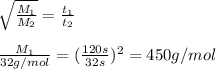Chemistry, 30.11.2019 01:31 hannahbeccahxo9681
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. it required 120 s for 1.0 l of the gas to effuse. under identical experimental conditions it required 32 s for 1.0 l of o2 gas to effuse. you may want to reference (pages 416 - 419) section 10.8 while completing this problem. part a calculate the molar mass of the unknown gas. (remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 l; that is, rate and time are inversely proportional.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

You know the right answer?
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure...
Questions

Business, 25.07.2021 23:00




Mathematics, 25.07.2021 23:00

Mathematics, 25.07.2021 23:00



History, 25.07.2021 23:00


English, 25.07.2021 23:00



Mathematics, 25.07.2021 23:00

English, 25.07.2021 23:00


Mathematics, 25.07.2021 23:00