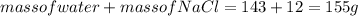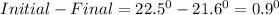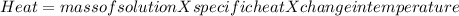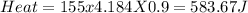1. a calorimeter contains 143 g of water at 22.5°c. a 12 g sample of nacl is added to the water in the calorimeter. after the solid has dissolved, the temperature of the water is 21.6°c. calculate the enthalpy of solution for dissolving sodium chloride. assume that no heat is lost to the calorimeter or the surroundings and that the specific heat of the solution is the same as that of pure water.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
1. a calorimeter contains 143 g of water at 22.5°c. a 12 g sample of nacl is added to the water in t...
Questions

Social Studies, 03.02.2021 07:50

Physics, 03.02.2021 07:50

Biology, 03.02.2021 07:50


Chemistry, 03.02.2021 07:50


Mathematics, 03.02.2021 07:50






Mathematics, 03.02.2021 07:50

Mathematics, 03.02.2021 07:50


Mathematics, 03.02.2021 07:50

Arts, 03.02.2021 07:50

Physics, 03.02.2021 08:00


Mathematics, 03.02.2021 08:00