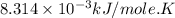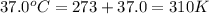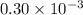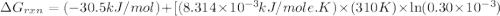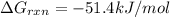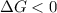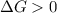Chemistry, 30.11.2019 01:31 redhot12352
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp(aq) +h2o (l) > adp(aq) +hpo4 (negative two overall charge) (aq).
for which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.30 mm, and [hpo4^2–] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Biology, 17.02.2021 23:00




Mathematics, 17.02.2021 23:00

Geography, 17.02.2021 23:00


Mathematics, 17.02.2021 23:00

Mathematics, 17.02.2021 23:00

Mathematics, 17.02.2021 23:00


Mathematics, 17.02.2021 23:00

Engineering, 17.02.2021 23:00


German, 17.02.2021 23:00

Computers and Technology, 17.02.2021 23:00




Mathematics, 17.02.2021 23:00

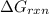 is -51.4 kJ/mol
is -51.4 kJ/mol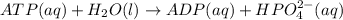
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0396/5636/ccdf0.png)
![[ATP]](/tpl/images/0396/5636/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0396/5636/68360.png) = 0.30 mM
= 0.30 mM![[HPO_4^{2-}]](/tpl/images/0396/5636/c0ca9.png) = 5.0 mM
= 5.0 mM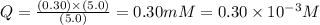
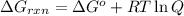 ............(1)
............(1)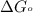 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol