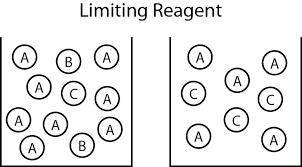
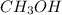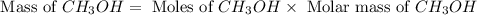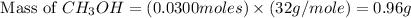Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2(g)→ch3oh(g) a 1.65 l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 374 mmhg .identify the limiting reactant and determine the theoretical yeild of methonal in grams.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2...
Questions


Mathematics, 20.11.2019 06:31

Biology, 20.11.2019 06:31


Chemistry, 20.11.2019 06:31


Mathematics, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

English, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31


Biology, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

English, 20.11.2019 06:31

Health, 20.11.2019 06:31

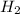 and the theoretical yield of methanol is, 0.96 grams.
and the theoretical yield of methanol is, 0.96 grams.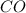 and
and 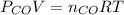
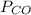 = pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)
= pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)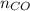 = number of moles of CO gas = ?
= number of moles of CO gas = ?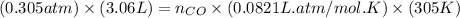
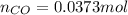
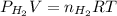
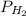 = pressure of
= pressure of 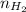 = number of moles of
= number of moles of 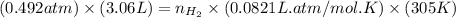
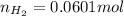
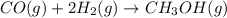
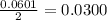 moles of
moles of