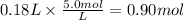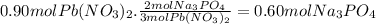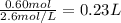Chemistry, 27.11.2019 22:31 marelinatalia2000
Assume 0.18 l of a 5.0 m solution of lead (ii) nitrate, pb(no3)2, reacts with a 2.6 m solution of sodium phosphate, na3po4, to produce lead (ii) phosphate, pb3(po4)2, and sodium nitrate, nano3. the problem requires that you determine the volume of sodium phosphate, na3po4, needed for the reaction to occur.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

You know the right answer?
Assume 0.18 l of a 5.0 m solution of lead (ii) nitrate, pb(no3)2, reacts with a 2.6 m solution of so...
Questions





Mathematics, 21.07.2019 18:30

Advanced Placement (AP), 21.07.2019 18:30

Mathematics, 21.07.2019 18:30




Chemistry, 21.07.2019 18:30


Biology, 21.07.2019 18:30


Spanish, 21.07.2019 18:30



Mathematics, 21.07.2019 18:30


Biology, 21.07.2019 18:30