
Chemistry, 27.11.2019 22:31 keishadawson
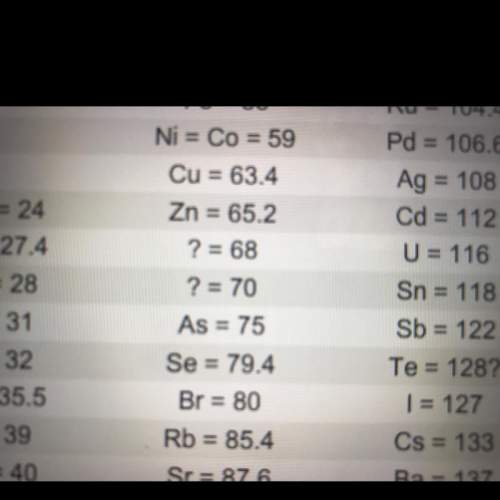
look at the two question marks between zinc (zn) and arsenic (as). at the time, no elements were known with atomic weights between 65.2 and 75. but mendeleev predicted that two elements must exist with atomic weights in this range.
what led mendeleev to predict that two undiscovered elements existed in that range?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
look at the two question marks between zinc (zn) and arsenic (as). at the time, no elements were kno...
Questions


History, 30.06.2019 20:30

Biology, 30.06.2019 20:30

Biology, 30.06.2019 20:30

History, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30




Physics, 30.06.2019 20:30



Mathematics, 30.06.2019 20:30



Spanish, 30.06.2019 20:30

Spanish, 30.06.2019 20:30

History, 30.06.2019 20:30



