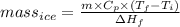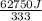Chemistry, 20.11.2019 20:31 jdsfdujfi1598
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitcher. if the tea is initially at 30.0°c and the ice cubes are initially at 0.0°c, how many grams of ice will still be present when the contents of the pitcher reach a final temperature? the tea is mostly water, so assume that it has the same density (1.0 g/ml), molar mass, heat capacity (75.3 j/k/mol), and heat of fusion (6.01 kj/mol) as pure water. the heat capacity of ice is 37.7 j/k/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1
You know the right answer?
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitche...
Questions


Mathematics, 15.06.2021 22:20

Mathematics, 15.06.2021 22:20


English, 15.06.2021 22:20

Mathematics, 15.06.2021 22:20



History, 15.06.2021 22:20

Biology, 15.06.2021 22:20


Biology, 15.06.2021 22:20

History, 15.06.2021 22:20




English, 15.06.2021 22:20



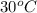 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K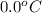 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K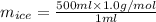
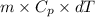
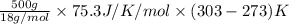
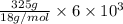
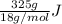 heat but we have 40774.95 J.
heat but we have 40774.95 J.