

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
Gthe following reaction is the first step in the production of nitric acid from ammonia. 4nh3(g) 5o2...
Questions



Chemistry, 26.11.2019 04:31




Geography, 26.11.2019 04:31



Chemistry, 26.11.2019 04:31

Mathematics, 26.11.2019 04:31





Biology, 26.11.2019 04:31

Mathematics, 26.11.2019 04:31

Mathematics, 26.11.2019 04:31


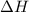
![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0383/2489/18a63.png)
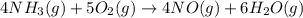
![\Delta H_{rxn}=[(4\times \Delta H_f_{(NO(g))})+(6\times \Delta H_f_{(H_2O(g))})]-[(4\times \Delta H_f_{(NH_3(g))})+(5\times \Delta H_f_{(O_2)})]](/tpl/images/0383/2489/02e37.png)
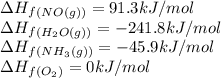
![\Delta H_{rxn}=[(4\times (91.3))+(6\times (-241.8))]-[(4\times (-45.9))+(5\times (0))]\\\\\Delta H_{rxn}=-902kJ](/tpl/images/0383/2489/6f6da.png)


