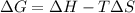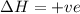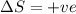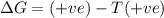Chemistry, 20.11.2019 20:31 Legoman29305
Consider a reaction that has a positive δh and a positive δs. which of the following statements is true? a) this reaction will be spontaneous only at high temperatures. b) this reaction will be spontaneous at all temperatures. c) this reaction will be nonspontaneous at all temperatures. d) this reaction will be nonspontaneous only at high temperatures. e) it is not possible to determine without more information.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Consider a reaction that has a positive δh and a positive δs. which of the following statements is t...
Questions

History, 22.05.2020 16:57

English, 22.05.2020 16:57


Mathematics, 22.05.2020 16:57

Mathematics, 22.05.2020 16:57

English, 22.05.2020 16:57

English, 22.05.2020 16:57

World Languages, 22.05.2020 16:57



Mathematics, 22.05.2020 16:57





Mathematics, 22.05.2020 16:57



Mathematics, 22.05.2020 16:57

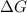 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous