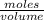Chemistry, 07.11.2019 22:31 nestergurl101
Then, calculate the molarity of a solution made by adding 5.3 g of cacl₂ to enough water to create a solution with a total volume of 2.50 l. enter only a numerical value in the correct number of significant figures. use the periodic table that is part of your exam to calculate molar mass. do not enter units in the answer box, but know that the symbol for the unit of this calculation is m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Then, calculate the molarity of a solution made by adding 5.3 g of cacl₂ to enough water to create a...
Questions

Chemistry, 05.07.2019 13:30


Biology, 05.07.2019 13:30

History, 05.07.2019 13:30

English, 05.07.2019 13:30

Social Studies, 05.07.2019 13:30




English, 05.07.2019 13:30


Mathematics, 05.07.2019 13:30

Social Studies, 05.07.2019 13:30





Biology, 05.07.2019 13:30

English, 05.07.2019 13:30

Biology, 05.07.2019 13:30