Chemistry, 07.11.2019 22:31 jerrica988
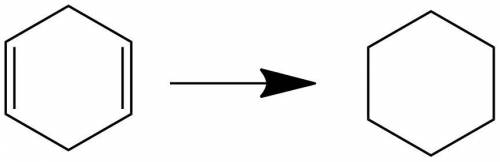
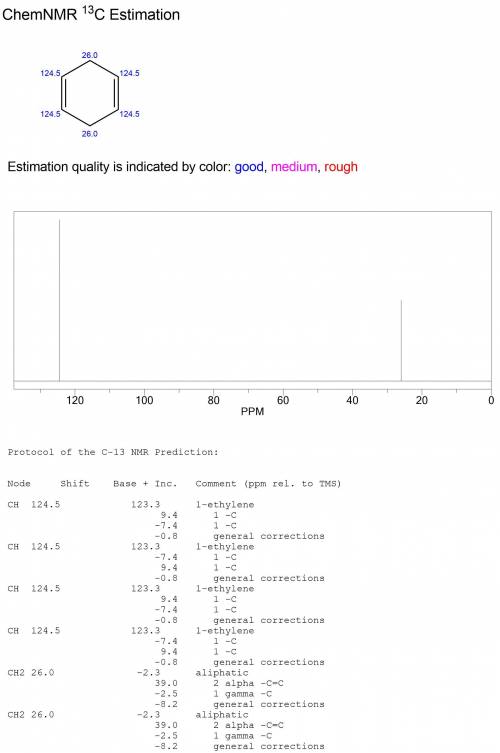
Compound o (c6h8) reacts with two molar equivalents of hydrogen in the presence of a catalyst to produce p (c6h12). the proton-decoupled 13c spectrum of o consists of two singlets, one at δ 26.0 and one at δ 124.5. in the dept 13c spectrum of o the signal at δ 26.0 appears as a ch2 group and the one at δ 124.5 appears as a ch group. note: all structures should be drawn with no bonds between carbon and hydrogen. draw the correct structure for o and p

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Compound o (c6h8) reacts with two molar equivalents of hydrogen in the presence of a catalyst to pro...
Questions

History, 27.04.2020 03:05


Mathematics, 27.04.2020 03:05


History, 27.04.2020 03:05

Mathematics, 27.04.2020 03:05


English, 27.04.2020 03:05





Mathematics, 27.04.2020 03:05

Health, 27.04.2020 03:05


English, 27.04.2020 03:05




Mathematics, 27.04.2020 03:06