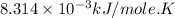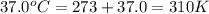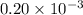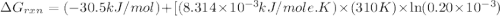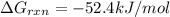Chemistry, 07.11.2019 22:31 brandon56238
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp(aq)+h2o(l)⟶adp(aq)+hpo2−4(aq) for which δ∘rxn=−30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ in a biological cell in which [atp]=5.0 mm, [adp]=0.20 mm, and [hpo2−4]=5.0 mm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions





Social Studies, 01.03.2021 06:30


History, 01.03.2021 06:30

Mathematics, 01.03.2021 06:30

Mathematics, 01.03.2021 06:30

English, 01.03.2021 06:30

Mathematics, 01.03.2021 06:30



Engineering, 01.03.2021 06:30

Mathematics, 01.03.2021 06:30

English, 01.03.2021 06:30

History, 01.03.2021 06:30



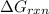 is -52.4 kJ/mol
is -52.4 kJ/mol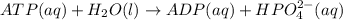
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0364/4024/ccdf0.png)
![[ATP]](/tpl/images/0364/4024/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0364/4024/68360.png) = 0.20 mM
= 0.20 mM![[HPO_4^{2-}]](/tpl/images/0364/4024/c0ca9.png) = 5.0 mM
= 5.0 mM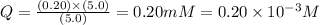
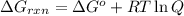 ............(1)
............(1)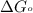 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol