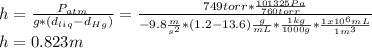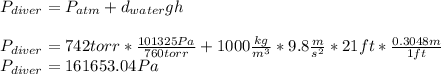Chemistry, 30.10.2019 03:31 jpichardo2021
The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20g/ml. the density of mercury is 13.6g/ml. what do you predict for the height of a barometer column based on 1-iodododecane, when the atmospheric pressure is 749 torr? what is the pressure, in atmospheres, on the body of a diver if he is 21 ft below the surface of the water when the atmospheric pressure is 742 torr?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20g/ml. the density of mercu...
Questions



Biology, 07.03.2020 01:24








Mathematics, 07.03.2020 01:24





English, 07.03.2020 01:24




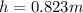
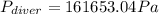
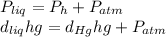
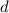 is the density,
is the density, 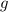 the acceleration of gravity,
the acceleration of gravity, 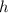 the height and
the height and 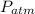 the atmospheric pressure, thus:
the atmospheric pressure, thus: