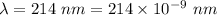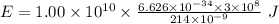Chemistry, 30.10.2019 03:31 lilyella1004
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there are 1.00×1010 atoms of zinc emitting light in the instrument flame at any given instant, what energy (in joules) must the flame continuously supply to achieve this level of emission? express your answer numerically in joules.
part b during an emission, electrons move from a higher energy orbital to a lower energy orbital. which of the following are valid transitions that produce lines in the emission spectrum of zn?
check all that apply.
1-[ar]4s13d106s1→[ar]4s23d10
2-[ar]4s23d10→[ar]4s23d104p2
3-[ar]4s23d10→[ar]3d10
4-[ar]4s23d10→[ar]4s13d11
5-[ar]3d10→[ar]4s23d10
6-[ar]4s23d10→[ar]4s13d106s1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if...
Questions




Mathematics, 12.03.2020 22:08

Chemistry, 12.03.2020 22:08


History, 12.03.2020 22:08





English, 12.03.2020 22:09




Computers and Technology, 12.03.2020 22:09




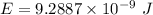
![[Ar]4s^13d^{10}6s^1\rightarrow [Ar]4s^23d^{10}](/tpl/images/0352/1669/c1431.png)
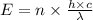
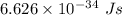
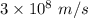
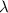 is the wavelength
is the wavelength